New EudraVigilance, the EU Medical Device Regulation (MDR), Brexit, AI, and automation technologies – these are just a few topics that are actively being discussed for the last year. At our recent stakeholder update meeting organized with Oracle in Milan, we looked at some of these trends closely: experts from Arithmos, its sister company seQure and Oracle presented regulatory changes in Medical Device vigilance, new technologies in signal detection, and change of demand in pharmacovigilance outsourcing.
We asked participants of the event, from pharmacovigilance to IT professionals of high seniority, to share their idea of the biggest challenges in pharmacovigilance, the most crucial trends and the main decision factors for choosing the right vendor. This is what we learned.
What is the biggest challenge in pharmacovigilance in your opinion?
Most of the participants find implementing an efficient safety system and database the biggest challenge. The second main challenge is ensuring quality in pharmacovigilance, which is followed by difficulties with the integration of the new technologies and coping with the regulatory landscape. The least widespread challenge is setting up an efficient signal system.

What is the most interesting Pharmaco- and Medical Device trend that you are following?
On the trends question the respondents are divided into three groups. The first and biggest group has chosen AI and Cloud technology as the most intriguing trend in the industry. However, during our stakeholder meeting revealed that most people are still unsure of the benefits of AI and how to manage it. The second place is divided between Medical Device Regulation and Automation technology.

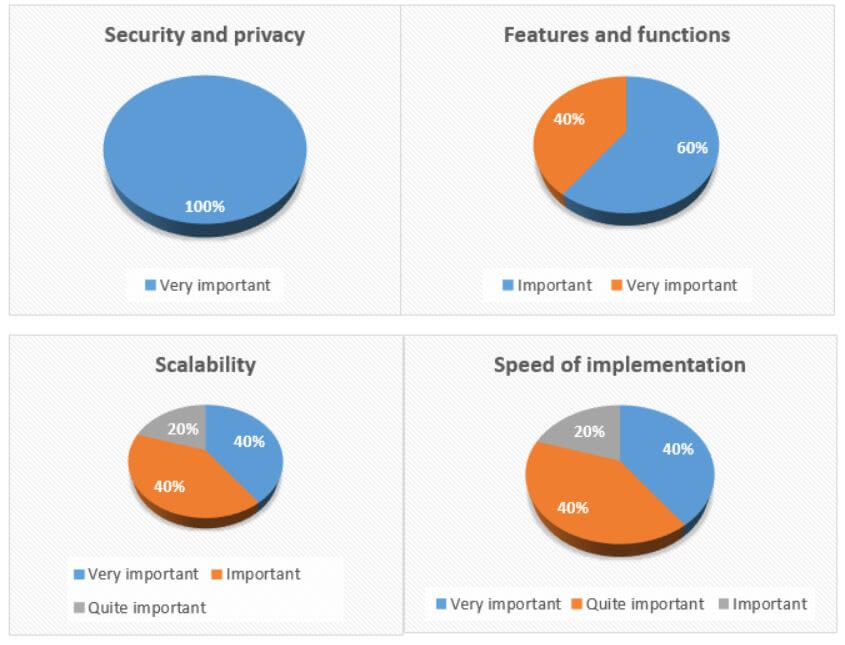
Which factors are the most important for you when it comes to a safety system solution?
Although the importance of different factors varies among the participants, the security and privacy factor was unanimously recognized as “very important”. Among other important factors are functionality, costs, scalability, and speed of implementation. This is one of the most significant changes we have seen over the past few years: the rise of concerns over data and information security and privacy. This further proves the importance of important security measures such as certifications, an Information Security Management System, and a comprehensive GDPR compliance roadmap.
Conclusion
With the pharmaco- and medical device industry evolving at high speed, trends and hot topics in the industry also change very quickly. However, as the survey shows, most of the topics and issues that concern the industry have persisted for years, such as AI in healthcare, MDR, and privacy and security.
The interests and concerns of the industry heavily shape vendor selection and oversight and safety system solution requirements. Solutions that combine efficiency, security, and quality are increasingly in demand on the market, yet they are not so easy to find.
Argus Blueprint, a pre-validated and preconfigured version of Oracle Argus Safety, is one of these solutions, as it guarantees the highest quality and efficient performance. Backed by an ISO 27001 certified ISMS, it responds to the challenges and demands of the pharmaco- and medical device market.
Want to learn more about Argus Blueprint? Book your free demo through the request form at the bottom of this product page!




