In Life Sciences industry, making sense of data becomes increasingly challenging due to its growing volume, introduction of new and complex technologies and increase of stakeholder numbers.
Introduction of the right data reporting and analytics solution at the corporate level allows Life Sciences companies not only to streamline the collaboration within the company by breaking down the silos between the departments but also to improve the decision-making process and optimise technology investments.
On July 8, Arithmos hosted a complimentary webinar on using data reporting and visualisation for breaking down the silos in Life Sciences companies. The webinar was conducted by Silvia Gabanti, Managing Director of Arithmos, and Massimo Businaro, CEO of E-project.
Continue reading to learn how effective data reporting and visualisation can improve the decision making at multiple levels, increase operational efficiency, and break the departmental silos.
Register now to get free access to webinar recording
Data Ecosystems in Life Sciences companies
Rapid technology advancement and new regulations benefit patients and speed up clinical trials. However, they also increase the amount of data generated daily. Real World Data, Internet of Things, ePRO – all these technologies allow to multiple the amount of data that a company deals with on a daily basis.
Data is the new fuel for Life Sciences businesses and source of competitive advantage; it drives innovation and stimulates growth.
Currently, despite the enormous potential of this newly generated data, a lot of pharmaceutical companies are suffering from the data silos issue. It manifests itself in an inability to share data across departments, the presence of multiple data repositories, and the absence of a clear picture of the company’s data.
As data is often stored in multiple tools and locations, data silos severely impact the departments’ ability to collaborate and harm scientific discovery and drug development.
Data silos are caused by multiple reasons:
- Every department has its own data repository isolated from the rest of the organisation
- Silos tend to arise naturally in organisations over time because each department or business function has different goals, priorities, responsibilities, processes, and data repositories.
- Legacy solutions don’t prioritise sharing data to other departments while maintaining data segregation, reduce the flow of information to what is considered strictly necessary
The challenge of data silo is normally recognised but often not addressed in a strategic and planned way, especially by small and medium companies.
Elimination of data silos and the creation of free data flow between the departments is the secret to transforming data into fuel for the company’s growth.
Breaking Down the Silos
If a pharmaceutical company plans to break down the data silos and use data to improve its performance and deliver its solutions to the market in a faster and more efficient manner, it should handle a cultural and structural change.
This can be done in 5 steps:
- Identify the cause of the company’s silo problem and the main impacted areas (ex. Pharmacovigilance department)
- Get management to buy in
- Define a scalable technology strategy that covers structural and cultural aspects of the change
- Embrace a corporate advanced reporting and analytics solution to create a bridge between the departments
- Invest in a process review and cross-functional training
Step 1: Identify the Cause of Silos and the Main Impacted Areas
Identifying the data management inefficiencies that impact company’s performance is very challenging. This is particularly true in small and medium companies without a Chief Data Officer who advocates the importance of collecting and leveraging data in decision-making.
In this case, the first step is to identify a business area that relies the most on data and rationalise the way it collects and manages information. This department will be the “Front door” of the change.
A good example is pharmacovigilance department. It is often perceived as a pure «cost» for the company, but that is key business unit in terms of regulatory compliance and patient safety.
Pharmacovigilance departments need to continuously improve their processes to reduce department costs. On the other side, they are under great pressure because of strict regulations and necessity to work closely with other departments (like regulatory affairs and clinical research) that have their own, completely different business goals.
Breaking down the data silos between pharmacovigilance and other departments eases the cooperation and allows faster and more robust decisions based on data analysis and visualisation, safeguarding patient’s health more efficiently.
Step 2: Get the Management to Buy In
To break down the data silos by introducing a cross-departmental reporting solution, you need to get the upper management to buy in.
Getting rid of data silos helps each individual department and the entire organisation by:
- offering them the big picture of the company’s data
- aligning company’s long-term goals and department objectives
- reducing the conflict between departments providing clear guidance on corporate priorities and avoiding conflict between personal objectives and corporate growth
- engage corporate stakeholders like IT which should be more conscious of the business goals and act as opinion leaders
Step 3: Define a Scalable Strategy
Pharmaceutical companies can find it challenging to decide which technology solution to choose or when to eliminate redundant technology.
This challenge can arise from biases in business decision making, which in turn can be caused by the following reasons:
- advocating previous choices (technologies, consultants, processes)
- sticking to the company’s previous habits
- lack of appropriate decision-support tools
To ensure the appropriate allocation of funds and minimal disruption to the company’s work during the period of change, it is critical to consider the change as a program. Optimisation of data management and data reporting should be included in a company’s defined roadmap.
This means that Work Breakdown Structure (WBS) approach should be followed – below you can find an example of WBS for defining activities of the project aim at breaking down the data silos.
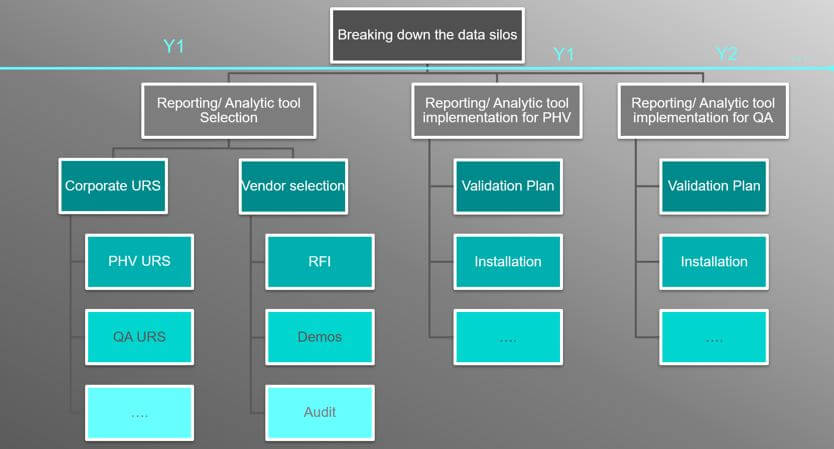
Step 4: Embrace Advanced Reporting and Analytics Tools
Most pharmaceutical companies manage different systems that contain heterogeneous and disparate data.
This gives rise to two challenges:
- Optimisation of the access to the information of the specific business systems (QA, PhV, RA databases)
- Increase of the ability to share data by rationalising and connecting these systems
Introduction of an advanced reporting and analytic solution allows pharmaceutical companies to:
- Support operational teams with data analysis and reporting and reduce manual elaboration of the data. This is not only time consuming, but also increases the risk of regulatory non-compliance
- Allow the managers to effectively oversee operational teams. This includes:
- Identifying challenging and ineffective processes
- Improving KPIs
- Obtain data that supports the decision-making process
- Integrate external data in a smooth manner
- Merge and evaluate data coming from different business areas to allow to identify in real time inconsistencies, carry out data reconciliation, avoid duplicating information between the systems, speed up the regulatory reporting, and increase data quality.
Step 5: Invest in Process Review and Cross-functional Training
Social change is a key step in breaking down the silos – all stakeholders must be fully committed to the successful result from the very beginning.
It is extremely important to share the company objectives both with management and operational teams. The management can define the strategic and economic advantages, but only the resources involved in the data management can identify the real bottlenecks that this solution can destroy.
The involvement of the teams during the requirements gathering, organisation of cross-functional workshops and training during the entire program development can improve the quality of the new processes and tools. What is more important, it can also break down the cultural silos (together with the data ones) reducing the change resilience and building relationships and corporate culture.
Conclusion
Companies can unlock the full data potential and optimize internal processes, and improve decision making by breaking down the data silos and allowing data to flow freely. Advanced data reporting and visualisation solution is a key step in this process alongside cultural and structural change.
The destruction of the data silos in Life Sciences companies can result in streamlined delivery of drugs to the market and more efficient work of all the departments, benefiting patients and allowing companies to safeguard their health more effectively.




